Grasp The Basics Of Clinical Trial Documentation
Grasp The Basics Of Clinical Trial Documentation
Published 9/2025
MP4 | Video: h264, 1280x720 | Audio: AAC, 44.1 KHz, 2 Ch
Language: English | Duration: 5h 1m | Size: 6.38 GB
Master the documentation and regulatory skills required to conduct clinical trials
What you'll learn
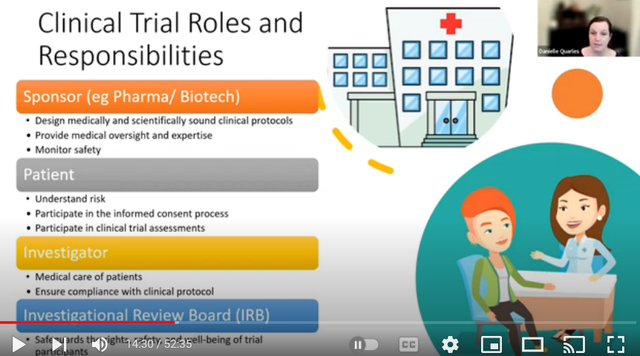
Gain in-depth knowledge of clinical trial documentation, regulatory requirements, and best practices in the industry
Training on study protocols and data management
Course provides you with the credentials and practical skills needed to secure a job in clinical research
Learn clear and effective communication with different stakeholders (investigators, sponsors, regulatory authorities, and trial participants) a crucial skill.
Required regulatory submissions for Clinical Trials in India
Requirements
Understanding of biology is the prerequisite
Description
This course covers the essential documentation required in clinical trials, including protocols, case report forms, and regulatory submissions with special reference to India context. Learn the importance of accurate and timely documentation in ensuring the integrity and validity of clinical trial data.Key Highlights:Protocols and Case Report FormsRegulatory SubmissionsEnsure Data IntegrityImportance of DocumentationCompliance with GuidelinesWhat you will learn:Understand Clinical Trial DocumentationLearn the basics of clinical trial documentation required in India and its significance in research studies.Create Comprehensive ProtocolsMaster the art of drafting clear and concise protocols for clinical trials.Complete Case Report FormsGain insights into accurately completing case report forms to capture essential data.Submit Regulatory DocumentsLearn the process of preparing and submitting regulatory documents for approvals.Ensure Data AccuracyUnderstand the importance of data accuracy and integrity in clinical trial documentation.Clear and effective communication with different stakeholders (investigators, sponsors, regulatory authorities, and trial participants) is crucial. Both written and verbal communication skills are essential for reporting, documenting, and coordinating tasks. Mastering these skills will equip candidates to excel in various roles within the clinical research industry, such as Clinical Research Associate (CRA), Data Manager, or Clinical Trial Coordinator.Dr. Pallavi Bafna brings years of expertise to guide you through this exciting career path. Don't miss this chance to learn from the best in the industry!
Who this course is for
Any life science graduate wish to join clinical trial industry in India
https://rapidgator.net/file/f3de3be8a2f4a81aed6f198f2531bc39/Grasp_the_basics_of_Clinical_Trial_Documentation.part1.rar.html
https://rapidgator.net/file/6aed184d5b1a0be3e57cfcece6717856/Grasp_the_basics_of_Clinical_Trial_Documentation.part2.rar.html
https://rapidgator.net/file/3d568a45a4dc8518686bb9c013f03494/Grasp_the_basics_of_Clinical_Trial_Documentation.part3.rar.html
https://rapidgator.net/file/530eb8756319d5ebbf1a47bade57a5f5/Grasp_the_basics_of_Clinical_Trial_Documentation.part4.rar.html
https://rapidgator.net/file/4c8130b9ad3dbcd2c0d52d06586afbb2/Grasp_the_basics_of_Clinical_Trial_Documentation.part5.rar.html
https://rapidgator.net/file/83c845ce890f24336be6e59a4838692c/Grasp_the_basics_of_Clinical_Trial_Documentation.part6.rar.html
https://rapidgator.net/file/61af57d263715b69148f9aaf524cbfb3/Grasp_the_basics_of_Clinical_Trial_Documentation.part7.rar.html

Information
Users of Guests are not allowed to comment this publication.



